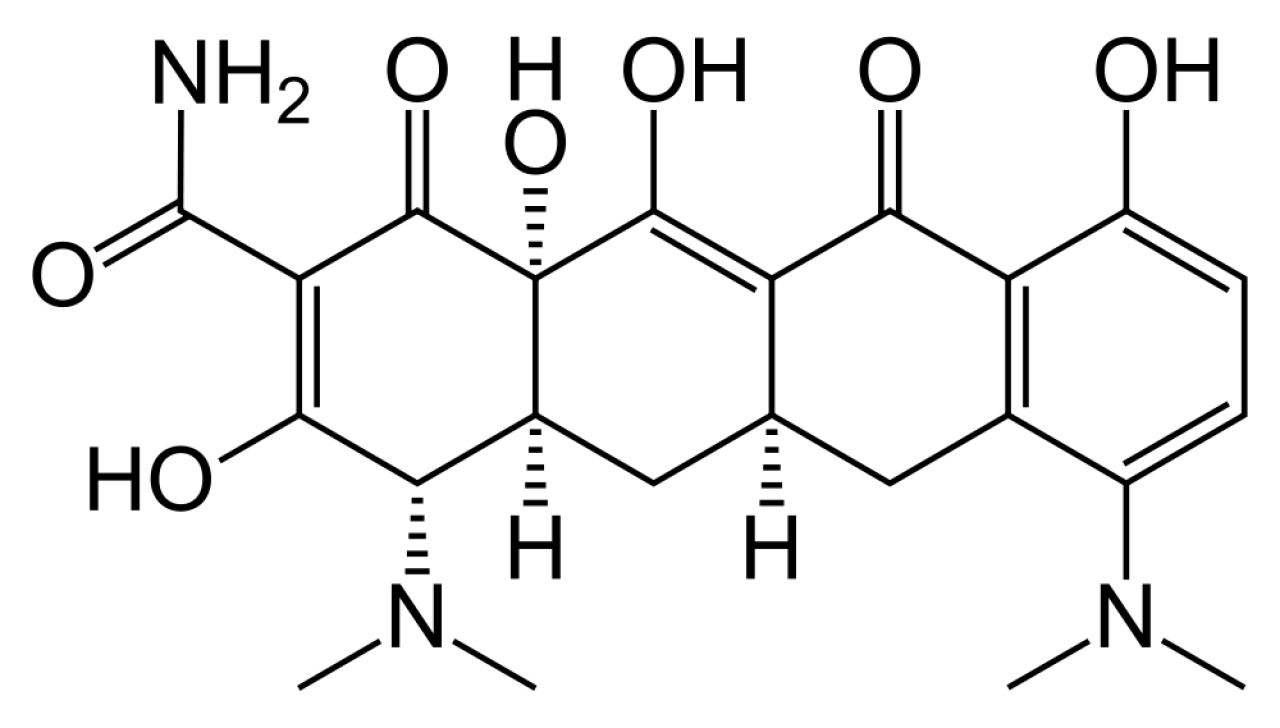
Minocycline Hydrochloride
API Product
Product Status:
Commercial
Available Grades:
- 未粉砕品
- 粉砕品
Regulatory Status:
日本 DMF
米国 DMF
欧州CEP
Production Sites:
- Hovione Loures(ポルトガル)
Product Type:
- テトラサイクリン系抗生物質
CAS Number:
13614-98-7
Modes of Application:
- 外用
- 抗感染症
- 注射
- 経口
Common Indications:
- 急性座瘡
Last Inspection:
FDA May 2018
Hovione is a leading supplier of Minocycline HCl since 1988 with an unblemished regulatory track record.
We have a long lasting strategic commitment to this product that ensures continuity an security of supply.
We can support new Minocycline HCl formulation development with a range of particle sizes that will meet your specific needs.
Hovione Minocycline is approved in generic and 505b2 applications.
This is not to be construed as a representation of non-infringement or as an offer to sell in those countries where such would constitute an infringement of third parties’ patent rights.
Highlight